Introduction
Cannabidiol (CBD) has rapidly become a star of the health and wellness world. It’s a non-psychoactive compound from Cannabis sativa touted for benefits in pain relief, anxiety reduction, inflammation control, and more. As CBD-infused oils, edibles, and topicals flood the market, there is growing interest in how this molecule produces such a wide range of effects. Understanding CBD’s molecular mechanisms is crucial – not only to validate its uses with science, but also to develop better CBD-based therapies and ensure safety by highlighting its beneficial effects through interactions with peroxisome proliferator-activated receptors (PPARγ) and its anti-inflammatory properties.
Scientists have discovered that CBD doesn’t act through one single pathway. Unlike THC (the intoxicating cannabis component which mainly activates cannabinoid receptors), CBD is often described as a “promiscuous” compound that influences many targets in the body (Molecular Targets of Cannabidiol in Neurological Disorders – PMC). It can bind to or modulate multiple receptors, ion channels, enzymes, and transporter proteins. By interacting with this network of molecular targets, CBD can tweak various biochemical pathways at once. This multi-target activity might explain CBD’s diverse therapeutic effects – but it also makes the science complex. In this article, we’ll break down the major molecular targets of CBD in a clear way. We’ll explore how CBD engages with specific receptors, transporters, ion channels, and enzymes, and discuss how these actions translate into potential health benefits like pain relief, anti-inflammation, anxiolytic (anti-anxiety) effects, neuroprotection, and more.
Why focus on molecular targets?
By examining CBD’s actions at the microscopic level – on cell receptors and proteins – researchers can connect the dots from chemistry to physiology. This helps answer important questions: How does CBD reduce a person’s pain or anxiety? Why might it help protect the brain or calm an inflamed immune system? Knowing the mechanisms also guides safe use of CBD alongside other medications, since CBD can influence drug-metabolizing enzymes in the liver. Overall, understanding CBD’s molecular targets bridges the gap between the growing popularity of CBD and the scientific rationale for its effects.
In the sections below, we delve into the key categories of CBD’s molecular targets: receptors, transporters, ion channels, and enzymes. For each, we highlight the most important examples and what happens when CBD interacts with them. We then consider how these molecular interactions might produce real-world therapeutic outcomes. All information is backed by scientific studies to ensure an engaging yet accurate exploration of how CBD works at the molecular level.
Receptor Targets of CBD: Cannabinoid Receptors
One way CBD exerts its effects is by interacting with various receptors in the body – proteins on cell surfaces (or inside cells) that trigger biological responses when activated or blocked. CBD is remarkably versatile: it influences not only the endocannabinoid receptors that THC targets, but also many other G protein-coupled receptors involved in pain signaling, mood regulation, and immune function. Below are some of the major receptor targets of CBD:
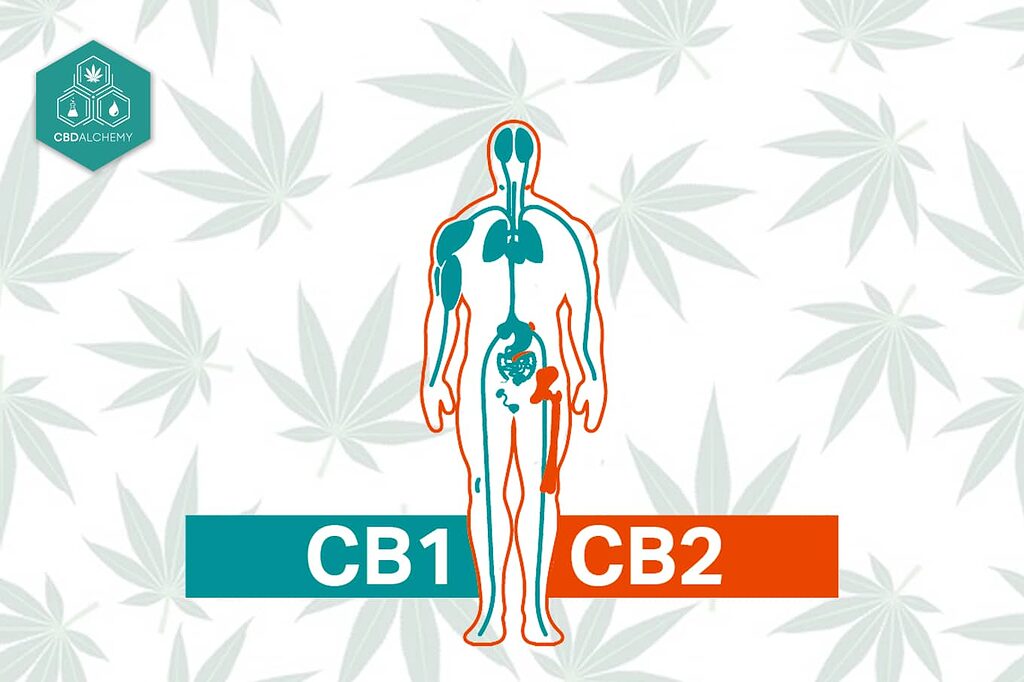
- Cannabinoid Receptors (CB1 & CB2) – These are the classic receptors of the endocannabinoid system. CB1 receptors are abundant in the brain (responsible for THC’s psychoactive high), while CB2 are found mostly in immune tissues. CBD has very low affinity for both CB1 and CB2, meaning it doesn’t fit well into the active “lock” site that endocannabinoids or THC bind (Molecular and Cellular Mechanisms of Action of Cannabidiol). In fact, CBD does not significantly activate CB1/CB2 directly, which is why it doesn’t cause intoxication. However, CBD can modulate these receptors in other ways. It acts as a negative allosteric modulator of CB1: CBD binds to a different site on the CB1 receptor and changes its shape, making it harder for other agonists (like THC or anandamide) to fully activate CB1. In simple terms, CBD dampens CB1 signaling, which might reduce side effects of THC or excessive endocannabinoid activity. CBD can also behave as an inverse agonist at CB2 receptors, subtly reducing CB2 activity. By influencing the cannabinoid receptors indirectly, CBD may balance the endocannabinoid system – for example, some effects (like neurogenesis, the growth of new brain cells) only occur if CB1 is present. Overall, CBD “tones down” CB1/CB2 receptor signaling rather than switching it on strongly.
- Serotonin Receptors (5-HT₁A subtype) – CBD interacts with the serotonin system, which is crucial for mood and anxiety regulation. Notably, CBD is an agonist at the 5-HT₁A serotonin receptor (the subtype 1A of serotonin receptors). In studies, CBD was shown to bind and activate 5-HT₁A receptors with micromolar affinity, triggering the associated cell signaling (it even increased the coupling of the receptor to its G-protein, a hallmark of agonist behavior). By stimulating 5-HT₁A, CBD can increase serotonin signaling. This is believed to contribute to CBD’s anxiolytic (anti-anxiety) and antidepressant effects, since 5-HT₁A activation produces calming and mood-lifting effects. For example, in animal models of anxiety, CBD’s effects were blocked when 5-HT₁A receptors were inhibited, confirming this as an important target. Beyond direct agonism, there’s also evidence CBD may act as a positive allosteric modulator at 5-HT₁A, meaning it can bind to a secondary site on the receptor to enhance the receptor’s response to serotonin itself. This multi-faceted interaction with the serotonin system is a key reason why CBD shows promise for conditions like generalized anxiety, social anxiety, and even nausea (5-HT₁A receptors in the brainstem are involved in vomiting reflex).
- Glycine Receptors (GlyR) – Glycine receptors are inhibitory receptors in the spinal cord and brainstem that dampen pain signals. Fascinating research has found that certain cannabinoids, including CBD, enhance glycine receptor function. CBD acts as a positive allosteric modulator of GlyR, which means when CBD binds to these receptors, it boosts their opening in response to glycine, increasing chloride ion flow into neurons and further inhibiting neuronal firing. In fact, a study showed that CBD can directly activate glycine receptors at higher concentrations and significantly potentiate (increase) the currents they produce. The relevance to pain is striking: in mouse experiments, cannabinoids’ analgesic effects were absent in mice lacking a specific glycine receptor subunit (α3 GlyR), but were intact in mice lacking CB1 receptors. This indicates that enhancing GlyR function is a major way cannabinoids reduce pain, independent of CB1. Thus, CBD’s potentiation of glycine receptors in the spinal cord can contribute to its ability to suppress inflammatory and neuropathic pain. This target is especially important because it suggests CBD could provide pain relief without engaging opioid receptors or causing sedation, by instead leveraging the body’s own inhibitory pain brake.
- Opioid Receptors (μ and δ) – The opioid receptors (mu, delta, and kappa) are well-known for their role in pain control, reward, and addiction. While CBD does not directly activate opioid receptors like morphine or endorphins do, it has been shown to allosterically modulate certain opioid receptors. Specifically, CBD is an allosteric modulator at the μ-opioid and δ-opioid receptors (). Research in cell assays demonstrated that CBD binding can change how these opioid receptors respond to their normal agonists. For instance, one study found CBD at micromolar concentrations could alter binding kinetics of radioligands to μ and δ receptors, accelerating the dissociation (unbinding) of opioid agonists from the receptor () (). In simpler terms, CBD may reduce the signaling intensity of opioid receptors by nudging them into an “less active” state or making opioid ligands detach faster. Notably, this was observed at concentrations higher than those typically achieved with normal CBD dosing in humans, so the in vivo significance is still unclear () (). However, this property is intriguing for two reasons. First, it’s shared by THC (THC also allosterically modulated opioid receptors in the same study) (), hinting that cannabinoids broadly influence pain pathways beyond the endocannabinoid system. Second, even a mild modulatory effect on opioid receptors might synergize with opioids or help in opioid-sparing pain management – some reports suggest patients using CBD need lower opioid doses for pain. More research is needed, but CBD’s interaction with opioid receptors represents another piece of the puzzle in pain and addiction contexts.
- Adenosine Receptors (A₁ and A₂A) – Adenosine is a neurotransmitter that generally has calming, anti-inflammatory effects (it’s the molecule that caffeine blocks to keep us alert). CBD has a significant impact on adenosine signaling, albeit indirectly. It increases extracellular adenosine levels by inhibiting its reuptake (more on that in the transporter section), which in turn means more activation of adenosine receptors A₁ and A₂A throughout the body. The result is that CBD enhances adenosine receptor signaling. Studies show that certain anti-inflammatory and cardioprotective effects of CBD disappear if you block A₂A adenosine receptors, indicating CBD works through these receptors. A₂A receptors on immune cells suppress release of inflammatory cytokines, so their activation by CBD can cut down inflammation. A₁ receptors in the heart can protect against arrhythmias during ischemia – indeed CBD’s adenosine-boosting effect can produce an anti-arrhythmic benefit via A₁ activation. It was initially unclear if CBD directly binds to A₁/A₂A receptors as an agonist; co-treatment experiments with adenosine antagonists suggested CBD’s effects were lost when those receptors were blocked. However, further pharmacological analysis indicates CBD is not a direct adenosine agonist, but rather works by blocking the adenosine transporter to raise adenosine levels (making it an indirect agonist). In summary, through boosting adenosine signaling, CBD can induce immunosuppressive and anti-inflammatory outcomes – a mechanism very different from cannabinoids’ action on CB receptors, but highly relevant for conditions involving inflammation and neuroprotection.
- Nuclear & Intracellular Receptors (PPARγ) – CBD can even influence gene expression by activating receptors located inside the cell nucleus. A prime example is PPARγ (peroxisome proliferator-activated receptor gamma), a nuclear receptor that regulates metabolism and inflammation. CBD is a direct agonist of PPARγ, binding to this receptor and increasing its transcriptional activity. When PPARγ is activated, it can switch on anti-inflammatory genes and antioxidant pathways. Researchers have found that CBD’s activation of PPARγ leads to reduced production of inflammatory cytokines like TNF-α and IL-1β, increased levels of anti-inflammatory IL-10, and inhibition of immune cell recruitment to inflamed areas. For example, in vascular endothelial cells, CBD (via PPARγ) lowered the expression of adhesion molecules (like VCAM-1) that cause immune cells to stick to blood vessel walls. In the brain, PPARγ activation by CBD protected neurons from beta-amyloid toxicity in Alzheimer’s models. PPARγ is also involved in insulin sensitivity and fat cell differentiation; while CBD’s metabolic effects via PPARγ are still being studied, this interaction suggests a role in metabolic health as well. It’s worth noting that various endocannabinoid-like compounds also activate PPARs, so CBD is tapping into a natural regulatory system for inflammation and metabolism. By turning on this nuclear receptor, CBD can produce longer-term changes in cell behavior, aligning with observed chronic effects like neuroprotection and reduced neuroinflammation. Aside from PPARγ, CBD may also antagonize another orphan receptor called GPR55 (often discussed as a “non-classical” cannabinoid receptor). GPR55 is a GPCR implicated in pain and inflammation signaling. CBD can block GPR55 activation (with an IC₅₀ around 0.45 μM in certain assays), which in rat hippocampal neurons was shown to restrict excessive excitatory transmission. This GPR55 blockade by CBD might contribute to its anticonvulsant and anti-inflammatory profile, though research is ongoing. Overall, CBD’s ability to engage intracellular targets like PPARγ (and possibly GPR55) sets it apart as a compound that doesn’t just act on the cell surface, but can penetrate inside and alter the cell’s programming in beneficial ways.
As we can see, CBD touches a remarkably diverse set of receptors: from the cell membrane (CB1, 5-HT₁A, glycine, opioid, TRP channels discussed later) to inside the cell nucleus (PPARγ). This broad receptor activity underlies many of CBD’s purported therapeutic effects. Next, we’ll look at how CBD affects transporter proteins that move neurotransmitters – another key part of its molecular toolbox.
Transporter Targets of CBD

Transporters are proteins that shuttle neurotransmitters and other molecules across cell membranes. They serve as gatekeepers, clearing neurotransmitters from synapses to reset signaling, or moving nutrients and messengers into cells. By interacting with transporters, CBD can alter the levels of various neurotransmitters and modulators in the brain and body. In fact, one of CBD’s most significant molecular actions is inhibiting certain transporter proteins, leading to increased levels of their substrates. Here are the major transporter systems influenced by CBD:
- Adenosine Transporter (ENT1) – CBD’s effect on adenosine is largely due to impeding its cellular uptake. Adenosine is removed from extracellular space by equilibrative nucleoside transporters (ENTs). CBD competitively inhibits ENT1, the main adenosine transporter, preventing cells from taking up and inactivating adenosine. Even at nanomolar concentrations, CBD has been shown to reduce adenosine uptake in various cell types (neurons, immune cells, cardiac cells). In one study, CBD displaced a radiolabeled ENT1 substrate with a K_i of ~237 nM, confirming a high affinity for this transporter. By blocking ENT1, CBD causes extracellular adenosine to accumulate and continually activate adenosine receptors (like A₂A on immune cells and A₁ in the heart). This mechanism explains why CBD can have anti-inflammatory and cardioprotective effects via adenosine signaling. It’s an indirect route – CBD doesn’t bind the adenosine receptor itself but makes more adenosine available to hit those receptors. ENT1 inhibition is one of CBD’s most potent molecular actions and likely contributes significantly to its immunosuppressive and anti-inflammatory properties.
- Serotonin Transporter (SERT) – There is evidence that CBD can inhibit the serotonin transporter, which would raise serotonin levels similar to how SSRI antidepressants work. Early studies with rat brain synaptosomes in the 1970s showed CBD (at high concentrations) could reduce uptake of serotonin (5-HT). At 50 µM CBD, about 78% of serotonin uptake was blocked in these preparations. However, at lower, more physiologically relevant concentrations (1 µM or less), the effect on SERT was not significant. This suggests CBD is a relatively weak SERT inhibitor unless at high dose. More recent research has been mixed: one ex vivo study found that 1 µM CBD did not affect serotonin uptake in rat synaptosomes, whereas another report indicated CBD might increase SERT expression or function in certain brain regions (complex, possibly indirect effects). In general, CBD is not as potent as SSRIs in inhibiting the serotonin transporter, but modest effects at high concentrations could still contribute to an antidepressant or anxiolytic profile. The more prominent serotonin-related action of CBD is through 5-HT₁A receptors (as discussed earlier), but SERT inhibition might give an extra boost to serotonin signaling when CBD is used in high doses or certain contexts.
- Noradrenaline (Norepinephrine) Transporter (NET) – Similar to serotonin, synaptosomal studies indicate CBD can inhibit the reuptake of noradrenaline. At 50 µM, CBD blocked ~81% of norepinephrine uptake in rat brain slices. Interestingly, a more recent experiment with rat hippocampal and striatal synaptosomes reported that even at 1 µM, CBD significantly inhibited noradrenaline uptake. This is notable because 1 µM is a concentration that might be reached in tissues with high CBD dosing. By inhibiting NET, CBD could increase noradrenaline levels in the synapse, potentially contributing to alertness or antidepressant effects (since SNRIs that block noradrenaline uptake have energizing antidepressant properties). However, boosting noradrenaline could also raise heart rate or blood pressure; yet in practice CBD tends to reduce anxiety and have mild hypotensive effects, so NET inhibition might be counterbalanced by other actions (like adenosine’s calming effect). Nonetheless, the ability of CBD to interfere with norepinephrine reuptake suggests it overlaps with mechanisms of some psychotropic medications, hinting at why CBD might help elevate mood or attention in certain cases.
- Dopamine Transporter (DAT) – Dopamine is the neurotransmitter of reward and motivation, and its transporter DAT is the target of stimulants like cocaine and amphetamine. CBD has been shown to modulate dopamine uptake as well. In rat striatal synaptosomes, CBD caused a dose-dependent reduction in dopamine uptake, with an IC₅₀ (half-maximal inhibitory concentration) around 16.2 µM. At 1 µM (again in synaptosomes), CBD significantly inhibited dopamine reuptake in both hippocampus and striatum. This suggests that fairly low concentrations of CBD might increase dopamine availability. Indeed, some studies in cell culture noted a transient decrease in DAT expression on the cell surface after CBD exposure, meaning less transporter available to clear dopamine (“Delta-9-Tetrahydrocannabinol and Cannabidiol Effect on Dopamine Transp” by Delia M. Guzman). The implication is that CBD could enhance dopaminergic signaling, which might contribute to its reported benefits in conditions like Parkinson’s disease (where dopamine is low) or addiction (modulating reward pathways). However, too much dopamine can also be associated with anxiety or psychosis – interestingly, CBD is being researched as an antipsychotic, possibly by balancing dopamine in nuanced ways. It’s hypothesized that CBD’s moderate inhibition of DAT, combined with its serotonin and adenosine effects, might produce a net anxiolytic/antipsychotic outcome rather than a stimulant one. In any case, DAT is clearly on the list of CBD’s targets, indicating CBD can influence the brain’s reward and motivation circuitry via dopamine.
- GABA Transporters (GATs) – GABA (gamma-aminobutyric acid) is the primary inhibitory neurotransmitter in the CNS, and increased GABA signaling has calming, anti-seizure effects. CBD’s impact on GABA transporters (which remove GABA from synapses) has not been as extensively characterized as other transporters, but early research gives some clues. The same 1970s synaptosome study found that at 50 µM, CBD inhibited GABA uptake by about 47% in rat brain slices. However, at 5–10 µM the effect was much smaller, and 1 µM did nothing significant. So CBD is a relatively weak GABA uptake inhibitor – certainly not as potent as typical GABA reuptake inhibitors (e.g., tiagabine). Nevertheless, combined with CBD’s direct GABA_A receptor modulation (CBD is an allosteric modulator of GABA_A receptors, see above), even a mild inhibition of GABA clearance could amplify inhibition in the brain. By allowing GABA to linger longer in the synapse, CBD might contribute to its anti-epileptic and anxiolytic effects. In fact, enhancing GABAergic tone is a common strategy in epilepsy treatment, and CBD is now an approved anti-seizure medication (Epidiolex). So while GABA transporters are not the primary target of CBD, they are part of the broad spectrum of proteins that CBD can influence at higher concentrations, potentially reinforcing the inhibitory/calming neurotransmission.
- Glutamate Transporters (EAATs) – Glutamate is the main excitatory neurotransmitter, and its excess can cause excitotoxicity (neuron damage). Glutamate is cleared by excitatory amino acid transporters (EAAT1-5). CBD appears to inhibit glutamate uptake in the brain at sufficient concentrations. In striatal synaptosome experiments, CBD reduced glutamate uptake in a dose-dependent way; however, it was less potent for glutamate than for dopamine, with an IC₅₀ around 43.8 µM. So it might take quite high CBD levels to significantly block EAATs. Why would we care about inhibiting glutamate uptake? One hypothesis is that during certain pathological conditions (like ischemia or trauma), preventing glial cells from hoarding glutamate could actually keep some synaptic activity going or activate protective pathways. But more likely, this effect is a secondary one. Interestingly, CBD also modulates glutamate release via other means (e.g., through adenosine and GPR55). Some studies in epilepsy models suggest CBD normalizes glutamate/GABA balance, but not simply by uptake inhibition. In summary, CBD can affect glutamate handling, but this is probably not its chief mechanism for neuroprotection given the relatively high concentrations needed. It’s a piece of the puzzle that shows CBD’s polypharmacy: it even touches the excitatory transmission system.
- Choline Transporter – The high-affinity choline transporter (CHT1) takes up choline into neurons for synthesizing acetylcholine (the neurotransmitter for cholinergic signaling). There have been inquiries into whether CBD influences cholinergic transmission. In vitro, CBD inhibited choline uptake in a rat brain slice preparation with an IC₅₀ of ~15.9 µM. However, in an in vivo experiment where rats were given a fairly large CBD dose (60 mg/kg), there were no significant changes in choline levels or uptake in various brain regions. This indicates that while CBD can block the choline transporter in a dish, it might not effectively do so in a living organism at tolerable doses. Thus, any impact on acetylcholine is likely minimal or indirect (CBD might still interact with muscarinic or nicotinic receptors, though, which is separate from transport). Given the mixed evidence, the cholinergic system is not considered a primary locus of CBD’s effects, but it underscores that CBD has been tested on essentially all major neurotransmitter transporter systems. The general takeaway is that CBD, especially at higher concentrations, has a broad inhibitory effect on neurotransmitter reuptake: increasing levels of adenosine, dopamine, norepinephrine (and to a lesser extent serotonin, GABA, glutamate, and acetylcholine) in synapses. This broad-spectrum transporter activity could underlie some of the synergistic effects of CBD – for instance, elevating both serotonin and adenosine yields anxiolysis, elevating dopamine and adenosine might yield neuroprotection, etc. Importantly, many of these findings come from in vitro studies using high doses; the challenge is translating what happens at realistic CBD doses in the human body. Nonetheless, the influence on transporters, particularly ENT1 (adenosine) and DAT/NET, highlights CBD’s capacity to fine-tune neurotransmitter levels and thus modulate mood, pain, and inflammation indirectly.
Ion Channel Targets of CBD
Ion channels are pores in cell membranes that allow ions (like Ca²⁺, Na⁺, K⁺) to flow in and out, which is fundamental for electrical signaling in nerves and muscle. The cbd functions include notable interactions with several ion channel families, affecting how excitable cells fire and communicate within the endocannabinoid system and its effects on potassium channels. Two major classes of channels that CBD targets are TRP channels and voltage-gated ion channels:
- Transient Receptor Potential (TRP) Channels – TRP channels are a large family of sensory ion channels that respond to temperature, pain stimuli, and various chemicals. CBD is particularly active on certain TRP channels, often called “ionotropic cannabinoid receptors” because they respond to cannabinoids. Specifically, CBD activates many of the TRP channels involved in pain perception: it is an agonist at TRPV1, TRPV2, TRPV3, TRPV4, and TRPA1, while acting as an antagonist at TRPM8. TRPV1 (the vanilloid receptor 1) is famous as the capsaicin receptor that mediates spicy heat and pain; CBD is a weak agonist of TRPV1, meaning it can trigger the channel to open and let ions through, although not as strongly as capsaicin. Initially, activating TRPV1 causes a burning sensation or pain, but it is typically followed by desensitization – the channel stops responding to further stimulation, leading to an analgesic effect. This is why capsaicin (chili extract) can paradoxically reduce pain after the initial sting. CBD’s activation of TRPV1 and TRPV2 on sensory neurons likely contributes to its reported analgesic and anti-inflammatory effects by desensitizing pain fibers. In inflammatory pain models, CBD’s anti-hyperalgesic properties were partly explained by TRPV1/TRPV2 interactions. Meanwhile, TRPV2-4 and TRPA1 are other pro-pain channels that CBD activates – these channels also contribute to inflammation and pain signaling, and their activation by CBD might lead to a similar desensitization and relief. TRPA1 (ankyrin receptor) is involved in noxious cold and chemical pain (like wasabi); including TRPA1, CBD engages at least six different TRP channels. On the flip side, TRPM8 is a cold-sensing channel (the menthol receptor) and CBD antagonizes/inhibits TRPM8. Inhibition of TRPM8 could be relevant to fighting certain types of pain or even inhibiting prostate cancer cell migration (TRPM8 is implicated in that). Overall, CBD’s broad modulation of TRP channels – often described as giving a warming or cooling sensation – underpins some of its sensory effects. Some topical CBD products for pain relief leverage this by combining CBD with terpenes that also target TRP channels. By hitting TRPV1 and related channels, CBD can trigger an analgesic cascade: first exciting the neurons, then silencing them (and releasing pain-relieving peptides). This TRP activity, combined with glycine receptor potentiation, puts CBD on multiple fronts of pain modulation.
- Voltage-Gated Calcium Channels (VGCCs) – These channels open in response to changes in membrane voltage and allow Ca²⁺ ions into cells, which is crucial for processes like neurotransmitter release and muscle contraction. CBD has been found to inhibit certain voltage-gated calcium channels, particularly the T-type Ca²⁺ channels (Cav3.1, Cav3.2, Cav3.3). T-type channels are low-voltage-activated calcium channels that help regulate neuronal firing rhythms and are involved in pain and absence seizures. In patch-clamp studies, CBD at micromolar levels was able to fully block T-type Ca²⁺ currents in cells expressing these channels. It showed similar potency for Cav3.1 and Cav3.2, and slightly less for Cav3.3. This was confirmed in native neurons (mouse trigeminal ganglion cells) where CBD reduced T-type calcium currents as well. Blocking T-type channels can dampen over-excitable neurons, providing a mechanism for CBD’s anticonvulsant effects and possibly contributing to pain relief (T-type blockers can have analgesic and even anti-hypertensive effects). Interestingly, some anti-epileptic drugs target T-type channels, and CBD’s efficacy in seizure disorders like Dravet syndrome might partly arise from this action. Beyond T-types, there is some evidence CBD might also affect other VGCCs (like N-type or P/Q-type which are involved in neurotransmitter release), but the most robust data is on T-types. By inhibiting VGCCs, CBD can reduce calcium influx into cells, which in neurons means less glutamate release (excess glutamate release is a problem in epilepsy and chronic pain). Indeed, a study noted that blocking T-type channels can reduce excessive glutamate release in the nucleus accumbens and produce antipsychotic-like effects. So, CBD’s calcium channel blockade might also tie into its anti-psychotic potential. In summary, CBD acts as a calcium channel blocker at sufficient concentrations, preventing the electrical overactivity of neurons by cutting down calcium entry, much like some existing antiepileptic and cardiovascular drugs (but via a different chemical structure).
- Voltage-Gated Sodium Channels (VGSCs) – Sodium channels are essential for the initiation and propagation of action potentials (electrical impulses) in nerves and muscles. CBD has been shown to block voltage-gated sodium channels as well, although with some interesting characteristics. Research using various models (brain slices, cultured neurons, transfected cells) found that CBD at micromolar levels can inhibit Na⁺ currents across multiple subtypes (Nav1.1, Nav1.2, etc.). However, the blockade observed didn’t follow a classic dose-response curve, suggesting it might be somewhat non-specific – potentially due to CBD’s ability to integrate into cell membranes (given its lipophilicity). In fact, one study pointed out that CBD (and another plant cannabinoid, cannabigerol) caused a sudden, steep block of sodium current that could be an artifact of membrane disturbance rather than a precise receptor binding. Nonetheless, more refined experiments, including X-ray crystallography and electrophysiology, have shed light on how CBD interacts with sodium channels. A 2020 eLife study crystallized CBD bound to a bacterial sodium channel and suggested a binding site, while a 2021 study on the human skeletal muscle sodium channel Nav1.4 found that CBD preferentially binds to the channel when it is in the inactivated state (Cannabidiol Selectively Binds to the Voltage-Gated Sodium Channel Nav1.4 in Its Slow-Inactivated State and Inhibits Sodium Current – PMC). Essentially, after a nerve fires, the sodium channel goes into a temporary inactive state; CBD stabilizes this state, making it harder for the channel to reactivate quickly. The binding affinity in that state was measured with a K_d of ~51 μM for Nav1.4, indicating CBD’s moderate potency. By stabilizing the inactive state of sodium channels, CBD prolongs the refractory period of neurons – which could prevent rapid, repetitive firing. This is relevant to conditions like epilepsy (uncontrolled neuronal firing) and maybe pain (hypersensitive nerves). Indeed, many local anesthetics and anti-arrhythmic drugs work by blocking sodium channels. CBD’s sodium channel blocking might not be extremely potent compared to pharmaceutical sodium channel blockers, but it could add to its anticonvulsant effect. However, it’s noteworthy that in a head-to-head comparison, another cannabinoid (CBG) also blocked Na channels in vitro but failed to show anti-seizure effects in animals, whereas CBD did show strong anticonvulsant effects. This implies that sodium channel block alone might not explain CBD’s anti-seizure activity; instead, a combination of targets (perhaps sodium channels plus GPR55, plus calcium channels, etc.) all contribute. Nonetheless, CBD’s interaction with Nav channels is a significant part of its profile, and there’s interest in whether derivatives of CBD could be developed as safer local anesthetics or muscle relaxants. Some patients anecdotally report that high-dose CBD can cause them muscle relaxation or slight numbness, which align with these channel effects.
- Other Ion Channels – Beyond the big categories above, CBD has a few other ion channel targets worth mentioning. It can activate TRPA1 (as noted with TRP channels), which is an ion channel but often grouped with TRPs. CBD may also modulate potassium channels indirectly – for example, by activating 5-HT₁A or adenosine A1 receptors, which then open certain K⁺ channels to hyperpolarize neurons (inhibiting firing). Additionally, there is some research that CBD interacts with mitochondrial ion channels such as the permeability transition pore or voltage-dependent anion channel (VDAC) on mitochondria, which could influence cell death pathways and calcium storage in cells. These are more specialized cases, but it underlines that CBD’s ion channel influence is widespread, from the cell membrane to organelles. In general, by modulating ion channels, CBD can alter the excitability of neurons and other cells, which is fundamental to its roles in seizure control, pain modulation, and even smooth muscle relaxation.
To sum up, CBD’s impact on ion channels means it can fine-tune the electrical signals in our nerves. It activates some channels (like TRPV1) to eventually desensitize them, and blocks others (like certain Ca²⁺ and Na⁺ channels) to prevent overactivation. This “electrophysiological” side of CBD complements its receptor and transporter effects – painting CBD as a compound that can both trigger and tranquilize cellular excitability as needed.
Enzyme Targets of CBD: Fatty Acid Amide Hydrolase
Beyond receptors and channels, CBD also interacts with enzymes – proteins that catalyze biochemical reactions. By inhibiting or altering enzymes, CBD can affect the levels of key signaling molecules and the metabolism of drugs. Several enzyme systems are known targets of CBD:
- Cytochrome P450 Enzymes (CYP450) – One of the most important (and clinically relevant) interactions of CBD is with the liver’s drug-metabolizing enzymes, the cytochrome P450 family. Pure CBD, which is non-psychoactive and distinct from THC, is a potent inhibitor of multiple CYP450 isoenzymes, which means it can slow the breakdown of many medications. For example, CBD strongly inhibits CYP2C19 (an enzyme that metabolizes drugs like clobazam, proton pump inhibitors, and some antidepressants) with a Ki around 0.8 µM. At a CBD concentration of just 10 µM, CYP2C19 activity can be almost completely shut down. This explains why CBD raises levels of certain antiepileptic drugs (like clobazam) – in clinical trials for epilepsy, some side effects were traced to CBD inhibiting clobazam’s metabolism causing higher clobazam levels. CBD also inhibits CYP2C9 (metabolizes NSAIDs, warfarin, etc.) with an IC₅₀ ~2.7 µM, and CYP2D6 (metabolizes many antidepressants and opioids) with IC₅₀ ~6 µM. Furthermore, CBD potently blocks enzymes in the CYP1 family: CYP1A1 (IC₅₀ ~0.5 µM) and to lesser extents CYP1A2 and 1B1 ( Molecular Targets of Cannabidiol in Neurological Disorders – PMC ,5)). It’s been reported that a low micromolar dose of CBD (2.5 µM) inhibited 90% of CYP1A1 activit. Lastly, CBD affects the major CYP3A family (which metabolizes ~50% of drugs). It inhibits CYP3A5 most potently (IC₅₀ ~1.65 µM), versus CYP3A4 at ~11.7 µM. About 90% of CYP3A5 activity was blocked by 10 µM CBD. Practically, this means if someone is taking CBD along with medications, those meds might not be cleared as fast, leading to higher blood levels. On the flip side, some have posited that inhibition of certain CYPs in the brain (if they’re present there) could augment neurotransmitter levels (for instance, inhibiting CYP2D6 in the brain might raise serotonin by slowing its breakdown). However, the main takeaway is: CBD is a strong inhibitor of drug-metabolizing enzymes, which is a double-edged sword – it can cause drug interactions, but it also indicates that at a molecular level, CBD is binding to and altering these enzyme proteins. This might contribute marginally to therapeutic effects (e.g., if CBD inhibits a brain enzyme that produces a toxin or metabolizes an endogenous neurochemical), but its clinical significance is mostly in pharmacokinetics. Physicians often advise caution with CBD if patients are on medications metabolized by CYP3A4, 2C19, etc. This aspect underscores that CBD, while natural, has potent biochemical activity on liver enzymes akin to a drug.
Therapeutic Implications
We’ve cataloged an impressive array of molecular interactions for CBD – but what do these mean for actual health outcomes? Here we connect the dots between CBD’s targets and its potential therapeutic effects on the body:
- Pain Relief: CBD’s analgesic effects are multi-pronged, thanks to its action on receptors and channels involved in pain pathways. By activating TRPV1 and related TRP channels, CBD ultimately desensitizes pain-sensing nerves, reducing their firing. Its positive modulation of glycine receptors in the spinal cord increases inhibitory signaling, which “turns down the volume” of pain transmission. CBD also indirectly raises anandamide levels (through FAAH inhibition and perhaps transporter effects), and anandamide can activate CB1 receptors in pain circuits to dampen pain perception. Additionally, CBD’s anti-inflammatory actions (explained below) reduce inflammatory pain at the source. Interestingly, CBD’s interaction with μ-opioid receptors allosterically () hints at synergy with endogenous opioids – while CBD alone doesn’t bind like morphine, it could enhance our own endorphin signaling or improve opioid pain relief when used together. In practice, animal studies have shown CBD can reduce both inflammatory pain (like arthritis) and neuropathic pain (nerve injury). Studies using a rat model have demonstrated how CBD influences behaviors such as anxiety and pain response, particularly emphasizing its interaction with serotonin receptors. The fact that cannabinoid analgesia persisted in CB1 knockout mice but not in glycine receptor knockout mice underscores that non-cannabinoid receptors (like GlyR) play a major role in cannabis-induced pain relief. This means CBD’s ability to act as a receptor agonist at GlyR could be pivotal for chronic pain conditions. On the inflammatory pain side, activating CB2 receptors via anandamide or PPARγ pathways leads to less swelling and nociceptor activation. Also, by blocking T-type calcium channels, CBD may prevent pain signals from summating. Clinically, many people use CBD topicals or tinctures for pain and report relief; while human studies are still catching up, the molecular basis for analgesia is clearly there, rooted in CBD’s receptor and channel activity.
- Anti-Inflammatory and Immune Modulation: Arguably, one of CBD’s strongest suits is its anti-inflammatory effect, which has implications for conditions like arthritis, autoimmune diseases, and even neuroinflammation in disorders like multiple sclerosis. CBD’s activation of Adenosine A2A receptors via increased adenosine is a powerful anti-inflammatory pathway: A2A activation on immune cells like macrophages and neutrophils suppresses the release of pro-inflammatory cytokines (e.g. TNF-α, IL-6) and inhibits immune cell migration. Indeed, CBD has been shown to lower TNFα and IL-6 levels in inflammatory conditions in vivo, an effect largely attributed to adenosine receptor engagement. Similarly, PPARγ activation by CBD leads to reduced expression of inflammatory genes (NF-κB-driven cytokines) and increased antioxidant genes. This nuclear receptor mechanism means CBD can actually shift the behavior of immune cells to a less inflammatory state. For example, in endothelial cells and microglia, CBD (through PPARγ and A2A) reduced the expression of adhesion molecules and chemokines that recruit leukocytes, thereby limiting inflammation in a model of MS. Furthermore, CBD’s inhibition of enzymes like IDO and iNOS helps to break the cycle of chronic inflammation and oxidative stress. CBD also directly suppresses NF-κB signaling, a master regulator of inflammation, possibly by preventing IκB kinase activation (some evidence suggests CBD keeps NF-κB inactive in the cytoplasm). In sum, CBD intervenes in the inflammatory process at multiple points: it reduces pro-inflammatory mediator production, increases anti-inflammatory signals (like IL-10 and adenosine), and prevents immune cells from over-activating. Therapeutically, this could translate to benefits in inflammatory bowel disease, rheumatoid arthritis, dermatitis, and other inflammatory conditions. In fact, preclinical models of arthritis show CBD applied to joints reduces inflammation and damage. People with autoimmune diseases have anecdotally reported improvements with CBD – biologically plausible given the above mechanisms. Importantly, CBD achieves this without broadly suppressing the immune system like steroids do; it tends to restore balance (homeostasis) rather than cause immunosuppression, which is ideal for chronic inflammatory disorders.
- Anxiety and Mood Disorders: The anti-anxiety (anxiolytic) effects of CBD are one of its most well-documented clinical benefits in human studies. Mechanistically, this is heavily tied to serotonin 5-HT₁A receptor agonism. As mentioned, 5-HT₁A activation produces anxiolytic and calming effects similar to buspirone (an anti-anxiety drug that targets the same receptor). CBD’s action on 5-HT₁A in the brain’s limbic regions (like the periaqueductal gray, amygdala, and prefrontal cortex) is thought to exert anxiolytic effects by reducing fear and stress responses. Additionally, CBD’s positive allosteric modulation of GABA_A receptors amplifies the brain’s main inhibitory tone , which helps control neural overactivity associated with anxiety. This is akin to how benzodiazepines work, though CBD’s effect is milder and non-sedating. In one study, CBD enhanced GABA_A receptor currents at low GABA concentrations, effectively making GABA more potent at its receptor. This could explain the relaxation and even sleep-improving effects people report with CBD, without heavily impairing cognition. Moreover, by inhibiting the uptake of anandamide and possibly serotonin/noradrenaline, CBD may elevate mood-related neurotransmitters. Higher anandamide (through FAAH inhibition) can cause a sense of calm and well-being via CB1 activation in stress-regulating circuits – for instance, anandamide in the ventral prefrontal cortex and amygdala can reduce learned fear responses. There’s evidence that blocking anandamide breakdown produces antidepressant-like effects, so CBD’s FAAH inhibition (though modest) might contribute to a mood boost. On top of that, CBD’s impact on dopamine could help anhedonia (inability to feel pleasure) often seen in depression by subtly enhancing reward signaling. A noteworthy point: in humans, a single dose of CBD has been shown to reduce anxiety during public speaking tests, and neuroimaging studies found changes in blood flow in anxiety-related brain regions consistent with an anxiolytic effect. Those align well with the 5-HT₁A and GABA mechanisms described. In depression models, CBD shows fast antidepressant effects possibly via increasing BDNF and serotonin signaling. The multi-target nature of CBD (serotonin, endocannabinoid, GABA, glutamate modulation) might be why it can affect mood without the lag time of typical antidepressants. All told, CBD’s therapeutic potential in anxiety and mood disorders is strongly supported by its receptor profile – acting like a balancer of the neurochemistry underlying stress.
- Neuroprotection and Neurodegenerative Diseases: CBD is being investigated for neuroprotective effects in conditions such as epilepsy, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. Several molecular targets contribute to these effects. Firstly, antioxidant and anti-inflammatory actions (via PPARγ, NF-κB inhibition, adenosine, etc.) protect neurons from chronic inflammation and oxidative damage, which are common threads in neurodegeneration. For example, in an Alzheimer’s model, CBD acting on PPARγ reduced inflammatory responses to β-amyloid and shielded neurons from toxicity. In Parkinson’s models, CBD’s antioxidant effect and possible activation of synaptic plasticity pathways have shown to reduce neuronal loss and improve motor behavior. Calcium channel blockade by CBD can prevent calcium overload in neurons during excitotoxic events (e.g., in stroke or seizures), thereby preventing cell death. Sodium channel modulation similarly can protect against hyperexcitable neuronal firing that damages circuits over time. A major success of CBD is in epilepsy (Dravet and Lennox-Gastaut syndromes) – its ability to reduce seizures is FDA-approved. This arises from a combination of the aforementioned ion channel effects (stabilizing neural membranes) and receptor effects (e.g., CBD’s antagonism of GPR55 receptors in the brain’s excitatory neurons appears to limit calcium release and excitatory transmission, providing an anti-seizure effect. Also, enhancing GABA_A receptor function and adenosine levels increases inhibitory tone, which is crucial in quelling seizures. In diseases like multiple sclerosis (MS), CBD’s suppression of microglial activation and inflammatory cytokines can protect myelin and neurons – a mouse study of MS found CBD treatment attenuated neuroinflammation and improved motor deficits, dependent on adenosine A2A receptors and reduced immune cell infiltration. Moreover, CBD’s interaction with the TRP channels may promote neuronal survival; for instance, mild activation of TRPV2 can trigger cytoprotective pathways in glial cells. Another intriguing target is TLR (Toll-like receptors) on immune cells in the brain – although not covered above, some research suggests CBD can diminish TLR4 signaling (a pathway that drives neuroinflammation in response to pathogens or cell debris). The collective effect of these molecular interactions is that CBD tends to create a more favorable environment for neurons: less inflammation, less oxidative stress, more inhibitory signaling, and support for cellular homeostasis. This bodes well for using CBD (or related cannabinoids) in neurodegenerative conditions, either alone or as an adjunct. It’s not a magic bullet, but it could slow disease progression or manage symptoms. For example, anxiety and insomnia often plague neurodegenerative disease patients; CBD can help those symptoms, indirectly benefiting cognitive function and quality of life.
- Other Health Conditions: CBD’s molecular tapestry opens the door to many applications. In substance use disorders, CBD’s impact on the dopamine and serotonin systems, as well as its anti-anxiety effect, might help reduce cravings and withdrawal anxiety. There is preliminary evidence CBD can reduce cue-induced craving in heroin addicts and help with nicotine quitting. Its ability to modulate opioid receptors allosterically and boost anandamide (which has reward-reducing effects) could make it a useful tool for fighting addiction. In cancer, CBD has shown anti-proliferative effects on tumor cells through mechanisms like activating TRPV2 (which can drive cancer cell death), promoting apoptosis via ROS production, and inhibiting migration by blocking GPR55 which is often overexpressed in tumors. CBD also can enhance the uptake of chemotherapy drugs by cancer cells by inhibiting drug-efflux pumps (it interacts with multidrug resistance transporters like P-glycoprotein). As an example, CBD inhibits the breast cancer resistance protein (BCRP), potentially helping to overcome chemotherapy resistance. In autism spectrum disorder, where preliminary trials are exploring CBD, the rationale includes CBD’s serotonin and GABA effects to reduce anxiety and improve social behavior, as well as its reduction of inflammation (there’s an inflammatory component in some autism cases). Cardiovascular health might benefit from CBD’s vasorelaxant and anti-arrhythmic properties: CBD’s activation of adenosine A1 receptors in the heart can protect against ischemia-reperfusion injury (by preventing arrhythmias), and its activation of TRPV1 in blood vessels causes vasodilation. Its PPARγ activation can also improve endothelial function and reduce atherosclerosis risk (PPARγ agonists are used for metabolic syndrome). Indeed, in diabetic rodents, CBD reduced the vascular damage and cardiac dysfunction associated with diabetes, attributed to anti-inflammatory and direct vessel-relaxing effects.
Overall, CBD’s therapeutic implications are broad but grounded in the molecular interactions we’ve discussed. It’s like a Swiss army knife that’s not extremely sharp on any one tool, but has many tools that together can address complex conditions with multiple underlying causes. Pain, for instance, isn’t just about one receptor – there’s inflammation, nerve sensitization, psychological aspects – and CBD hits several of those components at once (inflammatory enzymes, pain receptors, mood circuits). This polypharmacology is often beneficial for multifactorial conditions (most chronic diseases).
However, it also requires careful study to understand how these different actions interplay in a living organism. Encouragingly, many of CBD’s effects tend to complement each other towards a positive outcome (for example, reducing inflammation also helps pain and neuroprotection; lowering anxiety helps pain coping and reduces neuroinflammation, etc.). This network effect is why CBD is being explored in conditions ranging from psychiatric disorders and chronic pain to epilepsy and cancer.
What is Cannabidiol?

Definition and origin from the cannabis plant
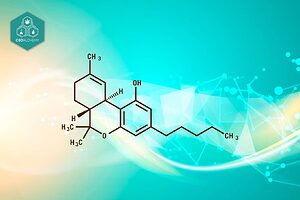
Cannabidiol (CBD) is a fascinating compound derived from the cannabis plant, specifically from its flowers, leaves, and stalks. It is one of the 113 identified cannabinoids in the cannabis plant, making up to 40% of the plant’s extract. Unlike its more famous cousin, Δ9-tetrahydrocannabinol (Δ9-THC), which is known for its psychoactive effects, CBD does not cause a high.
This non-psychoactive nature makes CBD an appealing option for those seeking the therapeutic benefits of the cannabis plant without the mind-altering effects. CBD can be extracted directly from the hemp plant, a variety of cannabis with low THC levels, or synthesized in a laboratory, ensuring a pure and consistent product.
Non-intoxicating effects
CBD has garnered significant attention for its potential health benefits, which are being explored in various scientific studies. Unlike THC, CBD does not produce intoxicating effects, making it a popular choice for individuals looking to alleviate symptoms without experiencing a high.
Research suggests that CBD may be effective in reducing anxiety, inflammation, and chronic pain.
It has also shown promise in treating certain types of epilepsy, such as Dravet syndrome and Lennox-Gastaut syndrome (LGS), where traditional medications have failed. Additionally, CBD is being investigated for its potential to improve sleep and manage conditions like insomnia.
Its non-intoxicating nature allows users to incorporate CBD into their daily routines without the concern of impairment, making it a versatile option for various health conditions.
Synthetic derivatives
The journey of CBD from a natural compound to a widely studied therapeutic agent is quite remarkable. Efforts to isolate the active ingredients in cannabis date back to the 19th century.
In 1940, researchers successfully studied cannabidiol from Minnesota wild hemp and Egyptian Cannabis indica resin, proposing its chemical formula.
Since then, the selective breeding of cannabis plants has expanded, driven by both commercial and therapeutic interests.
Today, numerous synthetic derivatives of CBD have been developed, offering similar therapeutic benefits.
These synthetic versions are meticulously researched for their efficacy and safety, providing an alternative to natural CBD extracts. As the market for CBD continues to grow, so does the diversity of its applications, from medical treatments to wellness products.
Legal Status and Safety
Is cannabidiol legal?
The legal status of cannabidiol (CBD) has been a topic of much debate and varies significantly across different regions. In the United States, the passage of the Farm Bill marked a significant milestone, removing hemp-derived products, including CBD, from the Controlled Substances Act.
This means that CBD derived from hemp is legal at the federal level, provided it contains less than 0.3% THC. However, the legality of CBD can still vary from state to state, with some states imposing stricter regulations than others.
Despite its legal status, the FDA does not currently regulate the safety and purity of dietary supplements, including CBD.
This lack of regulation means that the quality and concentration of CBD products can vary widely. Consumers should be aware of potential side effects, which may include nausea, fatigue, and irritability.
Additionally, CBD can interact with certain medications, such as blood thinners, anti-epileptics, and immunosuppressants, potentially increasing their levels in the blood.
It is crucial for individuals considering CBD to consult with a healthcare provider, especially if they are taking other medications, to avoid adverse interactions and ensure safe use.
Conclusion

CBD’s rise in popularity is backed by an increasingly detailed map of its molecular targets.
Unlike medical cannabis, which contains THC and other non-psychotropic cannabinoids, CBD operates through a network of receptors (CB1, CB2, 5-HT₁A, GlyR, TRPV1, etc.), transporters (for adenosine, dopamine, and others), ion channels (TRP channels, calcium and sodium channels), and enzymes (CYP450s, FAAH, COX/LOX, etc.).
By engaging this web of targets, CBD can influence pain signaling, inflammation, mood, and neuroprotection all at once. We saw that CBD can, for example, simultaneously reduce an immune cell’s inflammatory cytokine output (via A2A receptors and PPARγ) and increase an inhibitory neurotransmitter’s effect in the brain (via GABA_A receptors), while also prolonging the action of the body’s own endocannabinoid anandamide (by inhibiting its uptake and breakdown).
This holistic modulation is what gives CBD a sort of “regulatory” effect on the body – nudging imbalanced systems back toward equilibrium (homeostasis).
It’s important to note that many of CBD’s molecular interactions occur at relatively high concentrations in lab settings. Physiologically, some targets will be engaged more than others depending on the dose and route of administration.
For instance, a low dose might primarily hit 5-HT₁A receptors and adenosine transporters (yielding anxiety relief and a bit of anti-inflammation), whereas a very high dose might additionally block some sodium channels and inhibit LOX (potentially aiding seizure control and oxidative stress). This dose-dependent spectrum is a focus of current research.
Future studies are needed to fully map which targets are most relevant at therapeutic CBD levels in humans.
Another frontier is drug development: by understanding CBD’s key targets, researchers can design new molecules that might be more potent or selective at one of those targets for specific conditions.
For example, if FAAH inhibition by CBD is beneficial for anxiety, one could develop a CBD analog that’s a stronger FAAH inhibitor without affecting CYP450 (to avoid drug interactions). Or, if TRPV1 desensitization is the major pain-relieving component, scientists might create a peripherally-restricted CBD analog that targets TRPV1 in the body but doesn’t enter the brain (to avoid any sedation). Conversely, the knowledge that CBD hits so many targets is inspiring “multi-target” drug design – instead of one drug-one target, there’s a push for one drug-multiple complementary targets (as nature often provides). CBD is a case study in this modern pharmacology approach.
In conclusion, CBD’s molecular dance with our biology is complex but increasingly illuminated by science.
This cannabinoid exerts its effects by not one, but many molecular interactions, acting as a broad modulator of cell signaling. That quality is what underpins its diverse therapeutic potential – from easing pain and quelling inflammation to reducing anxiety and protecting neurons.
While more research will continue to refine our understanding (for example, uncovering any long-term adaptive changes from chronic CBD use, optimal dosing to engage desired targets, or discovering new minor targets of CBD), what is clear is that CBD represents a unique addition to the pharmacopeia: a single natural compound that can impact the endocannabinoid system, serotonergic system, and beyond, all at once.
As research forges ahead, we can expect to see more evidence-based applications of CBD in medicine, guided by this molecular insight. CBD’s journey from herbal remedy to scientifically validated therapy is well underway, and its rich pharmacology ensures that it will remain a fascinating subject for future discoveries in biochemistry and health.